Methods for Analyzing Chlorine: Which is Best?
Amperometric vs. Colorimetric
Chlorine is commonly used in various industries for various purposes, but they all need to monitor chlorine levels to ensure level are kept within safety margins. To ensure that the correct amount of chlorine is added to the process, two primary methods are used for chlorine analysis: colorimetric and amperometric methods. In this blog post, we will analyze each method, detailing how they work.
Collaborate with Modus Engineered Solutions
Improve operational efficiencies and compress time to market by leveraging innovation.
Colorimetric Method
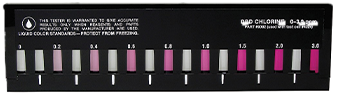
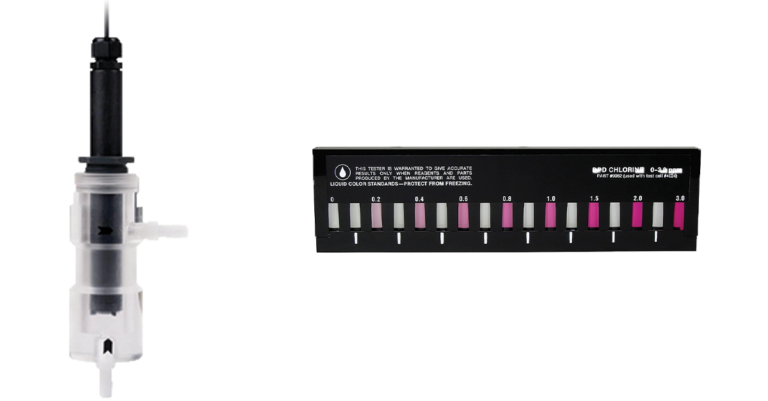
What is it?
In the colorimetric method, a colorimetric reagent such as DPD is added to a water sample that reacts with the chlorine present in the water to produce a colored compound. The intensity of the color change is then measured using a colorimeter or spectrophotometer, which can detect the specific wavelength of light absorbed by the colored compound. The intensity of the color change is proportional to the amount of chlorine present in the water sample, and this can be determined by comparing the color intensity of the sample to a calibration curve generated from standards of known chlorine concentration.
How Does It Work?
The colorimetric method can be performed using two different techniques: total residual chlorine (TRC) and free residual chlorine (FRC) methods. The TRC method measures the total amount of chlorine present in the water, including both free and combined chlorine. The FRC method, on the other hand, measures only the free chlorine, which is the portion of chlorine that is available to disinfect the water.
Considerations
The colorimetric method requires an additional buffer for the reading to take place. This requires procuring additional product regularly in order to gain a proper colorimetric reading. Although high levels of contaminants, like iron and copper, in the water cause readings to drift, colorimetric readings give accurate readings even with low amounts of chlorine present in the water. When compared to the amperometric method, the colorimetric method is as accurate at reading chlorine levels, but especially in applications where low levels of chlorine need to be detected, colorimetric does perform a little better. On the other hand, this method can be more costly to maintain as it requires the use of reagents which will have to continually be purchased in order for the readings to take place. Although the colorimetric methods does perform better in low level chlorine detection, the cost is much higher to maintain due to the need of reagents to be continually purchased.

Amperometric Method
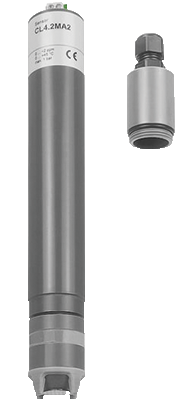
What is it?
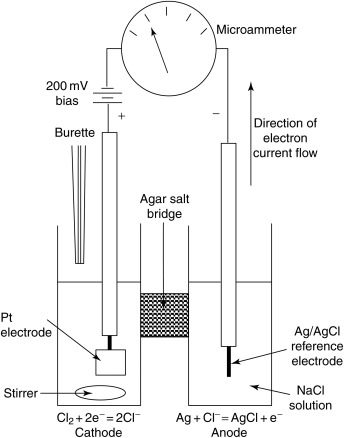
The amperometric method is based on the principle of electrochemistry. In this method, a sensor with a working electrode and a reference electrode is inserted into the water sample. The working electrode is typically made of a material that can be oxidized, such as gold or platinum. When the electrode comes into contact with the chlorine in the water, the chlorine is oxidized at the electrode, generating an electric current. The magnitude of the current is proportional to the amount of chlorine present in the water, and this can be measured using an amperemeter.
How Does It Work?
The amperometric method can be performed using two different techniques: direct measurement and indirect measurement. In direct measurement, the working electrode is exposed directly to the water sample, while in indirect measurement, a reagent is added to the water sample to react with the chlorine, generating a compound that can be oxidized at the electrode.
Considerations
Depending on the water quality, amperometric sensors life cycle and accuracy can be limited. If the water being sampled contains high level of iron, nitrite, sulfide, or other organic matter the sensor’s readings will drift and lifespan will shorten. It is also important to note that due to other chemicals or other matter in the water, regular maintenance, including calibration, is key to maintaining accurate results. When compared to the colorimetric, or DPD, method amperometric sensors are not sensitive to chlorine. Therefore, amperometric sensors should not be used in applications where lower levels of chlorine readings are required.
Collaborate with Modus Engineered Solutions
Improve operational efficiencies and compress time to market by leveraging innovation.
About Us
Modus Engineered Solutions is the preferred provider of critical service solutions. We leverage the vast experience and knowledge gained across our combined histories to provide exceptional solutions. We don’t just sell parts and service to our customers; we help them realize new and better ways to run more efficiently.
Copyright © Modus Engineered Solutions all rights reserved.
